Enhancing Customer Trust - The Crucial Link Between Quality and Compliance
Quality and compliance are closely interlinked as they both play a crucial role in earning and maintaining customer trust. By consistently delivering high-quality products that meet regulatory standards, Red Mesa Science & Refining demonstrates its commitment to meeting customer expectations and building trust.
Table of Contents
Prioritizing Quality, Transparency and Regulatory Compliance
Red Mesa Science & Refining ensures that every batch of CBD, CBG and CBN products meets the highest standards. Each regulatory requirement and other self-imposed stringent operational guidelines uniquely contributes to the highest purity, consistency and safety of our CBD, CBG, CBN and other minor cannabinoid products.
This post examines the relevance of cannabinoid raw ingredient regulatory compliance, the importance of a robust quality management system and the extra measures taken to ensure quality and complete transparency.
Implementing the following assures compliance to statutory and regulatory requirements while demonstrating a commitment to consistently providing products of the highest quality and value.

ISO 9001:2015 Certification
ISO 9001:2015 is an internationally recognized standard for quality management systems (QMS) developed by the International Organization for Standardization (ISO). It requires a systematic approach to managing quality within an organization and dedication to quality management systems.
ISO-certified, Red Mesa Science & Refining has consistently demonstrated the ability to provide products that meet both customer and regulatory requirements. Red Mesa has built a robust quality management system (QMS) to prioritize customer satisfaction, improve efficiency, manage risks and maintain a culture of continual improvement, ultimately building customer trust and achieving long-term success.
Why is being ISO 9001:2015 certified key to a brand’s product integrity and essential to choosing Red Mesa Science & Refining as a manufacturing partner?
ISO 9001:2015 sets the criteria for establishing and abiding by a comprehensive quality management system. It serves as the foundation to ensure the consistent delivery of quality products and services by including the following:
- Enhanced Customer Satisfaction – ISO 9001:2015 focuses on meeting customer requirements and exceeding expectations. For Red Mesa, our end-to-end ISO certification serves as a daily guide to improve customer satisfaction by providing consistent products of the highest quality, delivered promptly with an effective complaint-handling procedures if needed.
- Improved Efficiency and Effectiveness – ISO 9001:2015 emphasizes the need for well-defined processes, clear responsibilities and continual improvement. Adopting this standard helps us streamline operations, eliminate redundancies and identify areas for improvement, leading to increased efficiency, reduced waste and enhanced productivity.
- Risk Management – ISO 9001:2015 introduces a risk-based approach to quality management. It encourages us to identify and evaluate the risk of obstacles affecting the ability to deliver quality products. By proactively addressing risks, we can mitigate potential issues, improve decision-making and prevent costly errors or customer dissatisfaction.
- Increased Organizational Accountability – ISO 9001:2015 promotes a culture of accountability within Red Mesa. Establishing clear quality objectives, defining our team members’ roles and responsibilities and implementing performance measurement systems ensures that our employees understand their responsibilities and commitment to delivering quality products.
- International Recognition – ISO 9001:2015 is recognized globally and communicates a commitment to quality management. Red Mesa’s achievement in the end-to-end certification demonstrates to our customers, partners and stakeholders that we adhere to internationally recognized quality standards throughout our facility.
- Continuous Improvement- ISO 9001:2015 places a strong emphasis on continual improvement. As an organization, we monitor performance, collect and analyze data and make data-driven decisions to drive ongoing improvement. We are intently focused on continually enhancing our processes, products and services by implementing a cycle of planning, executing, checking and acting.
Current Good Manufacturing Practice (cGMP) Compliance
Similarly, implementing Current Good Manufacturing Practice standards ensures that products are consistently produced and controlled according to clearly defined quality standards. Following Current Good Manufacturing Practice guidelines requires establishing a robust quality management system and meeting minimum requirements for the methods, facilities and controls used in the manufacturing operations to ensure product safety, efficacy and quality in manufacturing. It is crucial for establishing consumer confidence, product consistency and continuous improvement.
All aspects of our production operation are addressed, from raw material acquisition, facility cleanliness and maintenance of equipment, to the training and personal hygiene of our staff. Implementing and following Current Good Manufacturing Practice standards demonstrates our commitment to consistently delivering high-quality, safe cannabinoid raw ingredients to consumers.
cGMP adherence is essential in industries that involve the manufacturing, processing, and packaging of pharmaceuticals, food and beverages, cosmetics and dietary supplements. cGMP adherence aligns with Red Mesa’s Quality system and is essential for the following reasons:
- Safety and Quality Assurance – Abiding by cGMP regulations ensures products are manufactured in a manner that promotes safety, efficacy and quality. Our commitment to complying with cGMP standards helps prevent product contamination, adulteration and defects. This guarantees consumers receive products that meet the highest quality and safety standards.
- Regulatory Compliance – Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), enforce cGMP adherence to protect the public’s health and ensure that manufacturers abide by established guidelines. Non-compliance can result in regulatory actions (including fines), product recalls and even the shutdown of organizations.
- Consumer Confidence and Trust – Conforming to cGMP is crucial for building consumer confidence and trust. When consumers purchase products, especially those related to their health and well-being, they expect them to be safe and high-quality. cGMP conformity assures consumers that manufacturers have followed stringent processes and procedures to deliver products that meet recognized quality standards.
- Product Consistency and Uniformity – cGMP adherence establishes standardized processes and controls to ensure consistent product quality and batch-to-batch uniformity. This is particularly important with cannabinoid raw ingredients, where efficacy and safety depend on consistent formulation.
- Supply Chain Management – cGMP conformity extends beyond the manufacturing facility to the entire supply chain. We, as Manufacturers, prioritize suppliers and contractors who adhere to the same cGMP standards to maintain overall integrity and safety throughout the production.
- Continuous Improvement and Risk Management – cGMP abidance requires companies to implement robust quality management systems and perform regular audits and inspections. Like ISO, this fosters a culture of continuous improvement, risk assessment and preventive action, helping identify and mitigate potential issues before they become significant problems.
- International Market Access – Many countries require adherence with cGMP standards for importing and exporting products. Abiding by cGMP standards allows Red Mesa to access global markets, expand our customer base and compete internationally.
The Importance of Understanding Jurisdictional Regulations
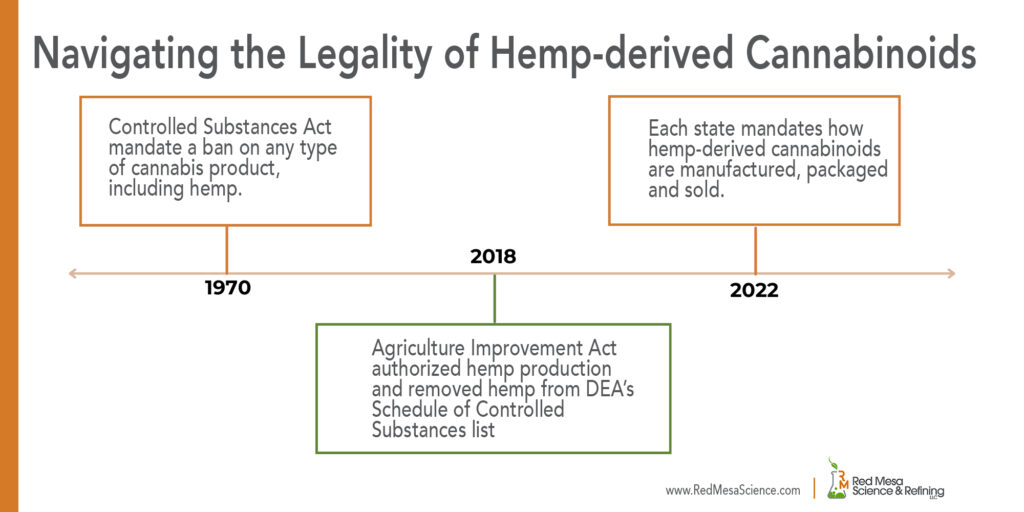
21 CFR Part 111 and 21 CFR Part 117 Adherence
21 CPR Part 111 (dietary supplements) and 21 CFR Part 117 (human food) are specific regulations concerning the manufacturing, packaging, labeling and holding of each and were established by the FDA to ensure the safety, quality, and proper labeling of dietary supplements and human food.
Located in Utah and it being one of two states requiring an additional layer of oversight, Red Mesa Science & Refining must abide by both 21 CPR Parts 111 and 117, even though hemp-derived CBD and other cannabinoids are yet to be regulated by the FDA as dietary supplements and human food. Additionally, while Red Mesa is not a direct manufacturer of food or dietary supplements, we are categorized as a food and dietary supplement manufacturer because we supply cannabinoid raw ingredients to food and dietary supplement product formulators.
Red Mesa embraces the additional regulatory requirements as it helps protect consumers, promotes comprehensive regulatory conformity and mandates the establishment of quality control measures. Here’s why choosing a manufacturing partner complying with these regulations is essential:
21 CFR Part 111 (Dietary Supplement Good Manufacturing Practices - GMPs):
- Safety and Quality Assurance – Abiding by 21 CFR Part 111 ensures that dietary supplements are manufactured, packaged, labeled and held in a manner that ensures their safety, identity, strength, purity and quality. These regulations establish good manufacturing practices specific to the dietary supplement industry, addressing areas such as personnel, facility, equipment, production and process controls, quality control, packaging, labeling and more.
- Consumer Protection – Complying with 21 CFR Part 111 is crucial for protecting consumers of dietary supplements from potential risks associated with adulterated or mislabeled products. These regulations set forth requirements for ingredient testing, identity verification, documentation and labeling accuracy to ensure that consumers can access reliable and accurately labeled dietary supplements.
- Regulatory Compliance – Complying with 21 CFR Part 111 is a legal requirement for manufacturers, packagers and labelers of dietary supplements in the United States. The FDA conducts inspections to assess abidance with these regulations and takes enforcement actions against non-compliant companies. Failure to comply can result in regulatory consequences, including product recalls, warning letters, fines and potential closure of organizations.
- Quality Control and Documentation – 21 CFR Part 111 mandates establishing quality control procedures, including written procedures, testing requirements and documentation of manufacturing processes. Adherence ensures that manufacturers have robust quality control systems to monitor and verify the quality of our cannabinoid raw ingredients, including testing raw materials, in-process materials and finished products.
21 CFR 117 (Food and Drink Food Manufacturing Practices – GMP):
- Food Safety and Preventive Controls – Adhering to 21 CFR Part 117 ensures that food manufacturers implement preventive controls to identify and minimize food safety hazards. These regulations require establishing a food safety plan, including hazard analysis, preventive controls, monitoring, corrective actions, verification and record-keeping.
- Regulatory Compliance – Complying with 21 CFR Part 117 is a legal requirement for organizations that manufacture, process, pack or hold human food for consumption in the United States. The FDA conducts inspections and takes enforcement actions against non-compliant companies. Failure to comply can result in regulatory consequences, such as product recalls, warning letters, fines and potential facility shutdowns.
- Consumer Safety and Confidence – Abiding by 21 CFR Part 117 helps protect consumers by establishing rigorous food safety standards. Adherence to these regulations ensures that food manufacturers have implemented robust preventive controls to address potential hazards, reducing the risk of foodborne illnesses and instilling consumer confidence in the safety and quality of the food they consume

FDA Registered Food Facility
Red Mesa Science & Refining is a FDA Registered Food company. Registered companies are important for regulatory conformity, food safety, access to global markets, recall communication, collaboration and consumer trust. It demonstrates our commitment to meeting food safety requirements and positions our facility as a reliable producer of safe, high-quality food products. In our industry, being an FDA Food Registered Facility is essential for several reasons:
- Regulatory Compliance – The Food Safety Modernization Act (FSMA) mandates that organizations engaged in the manufacturing, processing, packing or holding food for consumption in the United States must register with the Food and Drug Administration. Abiding by this requirement ensures that our company meets regulatory obligations and avoids potential penalties or enforcement actions.
- Enhanced Food Safety – Registering with the Food and Drug Administration demonstrates our commitment to food safety. The Food and Drug Administration uses registration information to identify and inspect food facilities to assess adherence to regulatory standards. By registering, we are signaling our willingness to adhere to good manufacturing practices (GMPs), hazard analysis, preventive controls and other food safety regulations, which ultimately help protect consumers from foodborne illnesses.
- Access to Global Markets – FDA registration can facilitate international trade by demonstrating conformity with regulatory requirements. Many countries and importers require Food and Drug Administration registration as a prerequisite for exporting or importing food products. Being an FDA-registered facility helps establish credibility, streamline customs processes and access global markets, helping expand our business opportunities.
- Recall Communication and Response – In the event of a food recall or safety alert, FDA registration enables effective communication between the FDA and registered facilities. The FDA can quickly identify registered facilities associated with the recalled product, ensuring timely and efficient communication, including the affected products, distribution channels and necessary actions. This helps protect public health by minimizing the distribution and consumption of potentially unsafe food products.
- Collaboration and Resources – Being an FDA-registered facility provides access to valuable resources and collaboration opportunities. The FDA offers registered facilities guidance, educational materials and training programs, helping to stay informed about regulatory updates, industry best practices and emerging food safety trends. Registered organizations can also participate in collaborative initiatives, such as partnerships for food safety prevention or research programs.
- Consumer Trust and Confidence – FDA registration symbolizes our commitment to transparency and the conformity of regulatory requirements. Being an FDA-registered facility enhances consumer trust and confidence in the safety and quality of the food ingredients we produce. It demonstrates our dedication to following industry best practices and meeting rigorous food safety requirements, which is particularly important in an era of increased consumer awareness and demand for safe and reliable food.
ISO 17025 Accredited Third-Party Lab COAs
Red Mesa provides ISO 17025 accredited third-party lab COAs to instill the utmost confidence and trustworthiness in final full panel third-party lab results. Obtaining COAs from ISO 17025 accredited third-party labs is vital for ensuring our CBD products’ reliability, accuracy, compliance, consumer trust and industry credibility. It is a proactive measure that demonstrates our commitment to delivering safe and high-quality products to the market.
ISO 17025 accreditation ensures that testing laboratories have the competence to carry out specific tests, providing a level of assurance of the reliability of their results. As a manufacturer and formulator of CBD products, it is crucial to obtain Certificate of Analysis (COAs) from ISO 17025 accredited third-party labs for several important reasons:
- Reliable and accurate results – ISO 17025 accreditation ensures that the third-party lab has rigorously been evaluated for its technical competence and proficiency. These labs follow internationally recognized standards and practices, guaranteeing reliable and accurate testing methods. By obtaining COAs from accredited labs, our customers can have confidence in the accuracy of the results, ensuring the quality and safety of their CBD products.
- Testing using recognized standards – The cannabinoid industry is subject to various regulations and standards. Compliance is essential to ensure consumer safety and avoid legal issues. COAs from ISO 17025 accredited labs demonstrate that our products have been tested following recognized standards, enhancing compliance and reducing the risk of non-compliance penalties.
- Consumer trust and transparency – Third-party COAs from ISO 17025 accredited labs provide transparency and build consumer trust. They demonstrate that Red Mesa is committed to quality and safety by independently verifying our CBD products’ potency and absence of impurities. Providing COAs upon request before purchase demonstrates our dedication to transparency.
- Industry credibility and competitive advantage – Submitting samples to an ISO 17025 accredited third-part labs strengthens Red Mesa’s brand credibility within the CBD industry. It demonstrates our commitment to quality, safety, and compliance, setting us apart from others who may not prioritize ISO 17025 accreditation.
Experience Excellence Beyond Expectations with Red Mesa Science & Refining
In our rapidly evolving industry, ensuring high-quality raw ingredients isn’t just a priority—it’s an absolute necessity. At Red Mesa Science & Refining, we don’t merely meet necessities; we seek to exceed them while at the same setting the standard for quality, compliance and innovation.
In closing, choosing a manufacturing partner shouldn’t be impulsive but rather done so with intention. A deep dive into a prospective manufacturing partner, their quality management system and conformity to statutory and regulatory requirements is paramount to supporting a brand’s reputation. As such, Red Mesa Science & Refining is fully transparent in its operation and encourages site visit evaluations to demonstrate firsthand why Red Mesa should be the manufacturing partner of choice


